"I can't
imagine anything more heart-wrenching than believing that you or a
family member could be helped by something out there but you can't get
it," says Weinstock. The FDA is in a difficult situation, he adds,
because "they want to be as aggressive as possible in allowing people to
have access to as many drugs as possible."
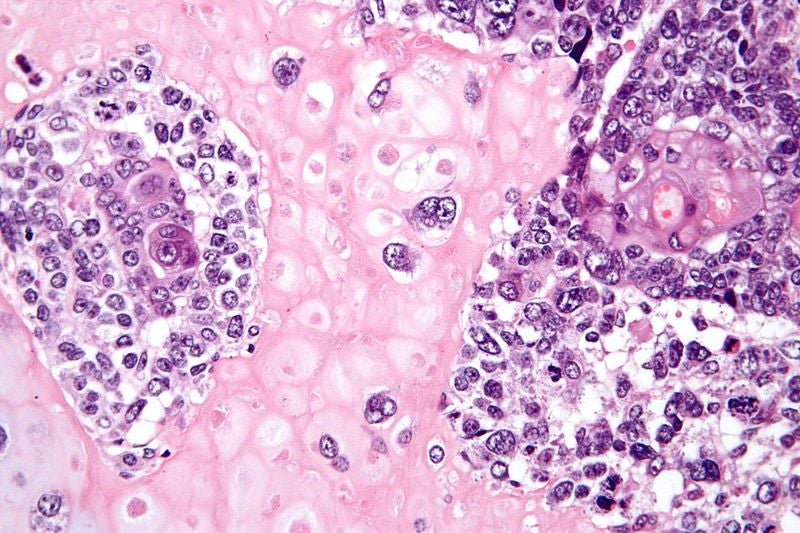 Expand
Expand
There's a
misconception that the FDA is a bunch of bureaucrats, making arbitrary
decisions based on politics — but Weinstock says it's actually "people
like me, academics and scientists and clinicians, who get asked to serve
on committees... and the overwhelming majority of the time, the FDA
goes along with [our] decisions." Image via Nephron.
There are
plenty of drugs that do a great job of shrinking tumors in mice, but
turn out not to be suitable for humans, adds White. "Obviously you're
excited [when a drug works on mice]. But the problem is that 95 percent
of those new drug applications fail. They don't get FDA approval. 70
percent of them fail in that Phase One, which is the toxicity study.
That leaves with you with 30 percent. And then 60 percent of those fail,
and [most] of the time, it's because they don't work. That's nobody's
fault. It isn't surprising that progress is slow."
"It's a
tragedy when the drug is toxic and hurts people, and it's almost as
tragic when the drug doesn't work," says Weinstock. "We've seen that
recently with Avastin," which had its approval for breast cancer
revoked.
"Very good
people and very smart people spend their life trying to beat this
disease," says White. "So every time you see a glimmer of hope, you hope
that this is the great breakthrough... and obviously that gets hyped."
Part of this is because you constantly have to seek more funding for
your research, and everyone from members of Congress to your peers want
to fund research that looks exciting. And then, of course, there's the
media — and we certainly tend to get over-excited as well.
No comments:
Post a Comment